 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
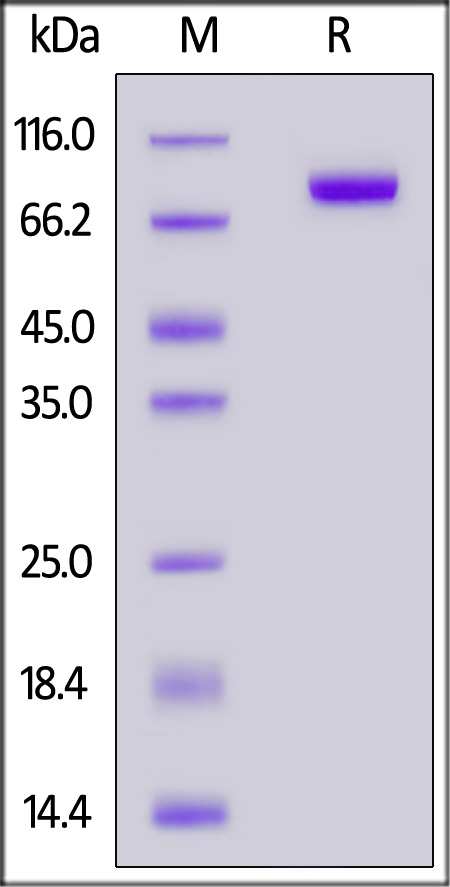
| FG4-H5253 | Human | Human FGF R4 / CD334 Protein, Fc Tag (MALS & SPR verified) |  |
|
|
| FG4-M52Ha | Mouse | Mouse FGF R4 / CD334 Protein, His Tag |  |

|
|
| FG4-H5228 | Human | Human FGF R4 / CD334 Protein, His Tag |  |

|

Human FGF R4, Fc Tag (Cat. No. FG4-H5253) immobilized on CM5 Chip can bind Human FGF-4 Protein, His Tag (Cat. No. FG4-H51H3) with an affinity constant of 1.48 μM as determined in a SPR assay (Biacore 8K) (QC tested).
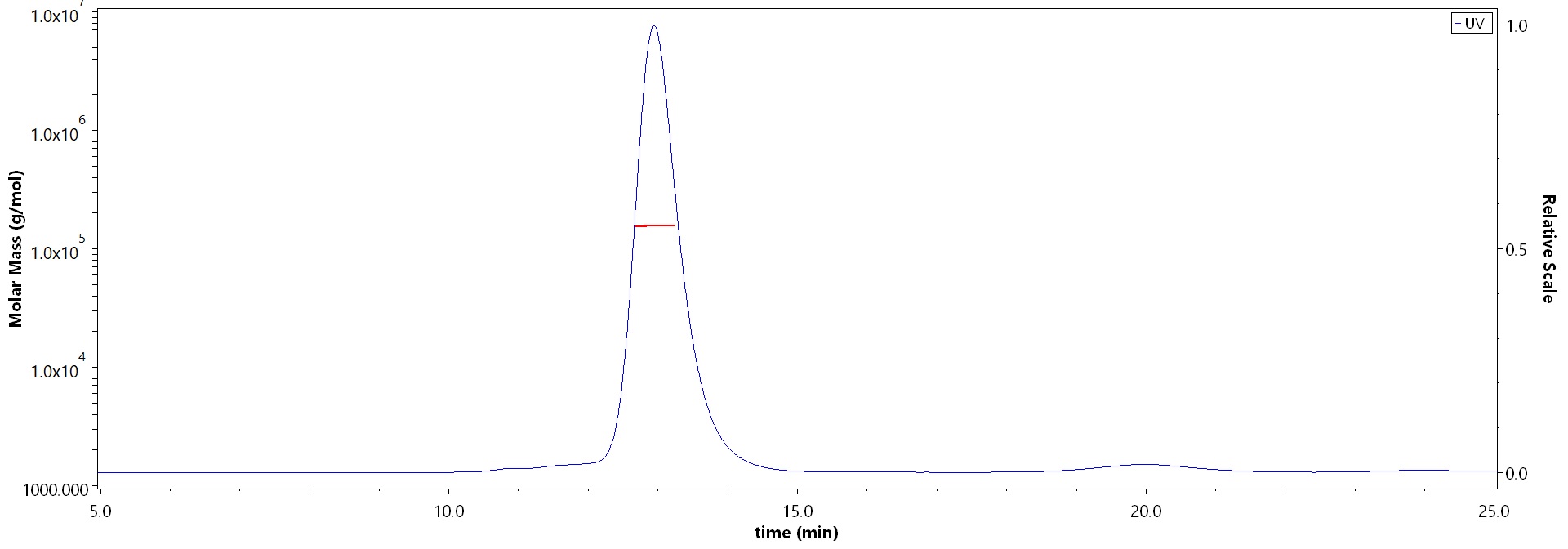
The purity of Human FGF R4, Fc Tag (Cat. No. FG4-H5253) is more than 90% and the molecular weight of this protein is around 145-175 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Solid tumours; Bone Marrow Neoplasms; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Central Nervous System Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Sarcoma; Lymphoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Lenvatinib Mesylate | ER-203492-00; E-7080; MK-7902 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | Japan | Carcinoma, Renal Cell | Merck Sharp & Dohme Corp | 2015-02-13 | Liver Diseases; Paraganglioma; Neoplasm Metastasis; Thyroid Cancer, Papillary; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Osteosarcoma; Cholangiocarcinoma; Breast Neoplasms; Solid tumours; Neuroendocrine Tumors; Carcinoma, Adenoid Cystic; Adenocarcinoma, Follicular; Kidney Diseases; Neoplasms; Adenocarcinoma of Lung; Thyroid Carcinoma, Anaplastic; Pheochromocytoma; Renal Insufficiency; Esophageal Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Erdafitinib | 890E37NHMV; JNJ-42756493; G-024; JNJ-493 | Approved | Astex Pharmaceuticals Inc | Balversa | South Korea | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Neoplasms, Neuroepithelial; Osteosarcoma; Neuroblastoma; Breast Neoplasms; Urologic Neoplasms; Hepatic Insufficiency; Histiocytic Sarcoma; Bile Duct Neoplasms; Lymphoma; Lymphoma, Non-Hodgkin; Sarcoma; Glioma; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Xanthogranuloma, Juvenile; Neoplasms, Germ Cell and Embryonal; Neuroectodermal Tumors, Primitive, Peripheral; Rhabdoid Tumor; Hematologic Neoplasms; Rhabdomyosarcoma; Ependymoma; Medulloblastoma; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma; Hepatoblastoma; Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Wilms Tumor; Multiple Myeloma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Histiocytosis, Langerhans-Cell; Sarcoma, Ewing | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| BPI-43487 | BPI-43487 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Biliary Tract Neoplasms; Solid tumours; Carcinoma, Hepatocellular | Details |
| HS-10340 | HS-10340 | Phase 1 Clinical | Changzhou Hengbang Pharmaceutical Co Ltd, Shanghai Hansoh Biomedical Co Ltd, Jiangsu Hansoh Pharmaceutical Group Co Ltd | Solid tumours | Details |
| JAB-6343 | Phase 1 Clinical | Jacobio Pharmaceuticals Co Ltd | Solid tumours | Details | |
| LY-2874455 | LY-2874455 | Eli Lilly And Company | Details | ||
| INCB-62079 | INCB-62079; INCB-062079 | Incyte Corp | Details | ||
| EVER-4010001 | EVER-4010001 | Phase 2 Clinical | EverNov Medicines (Zhuhai Hengqin) Co Ltd | Solid tumours | Details |
| ZSP-1241 | ZSP-1241 | Phase 1 Clinical | Guangdong Zhongsheng Pharmaceutical Co Ltd, Wuxi Apptec Co Ltd | Biliary Tract Neoplasms; Liver Neoplasms; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Colorectal Neoplasms | Details |
| U3-1784 | U3-1784 | U3 Pharma | Details | ||
| ODM-203 | ODM-203 | Orion Corp | Details | ||
| ICP-105 | ICP-105 | Phase 1 Clinical | Nanjing Tianyin Jianhua Pharmaceutical Technology Co Ltd, Beijing Tiancheng Pharmaceutical Technology Co Ltd, Beijing Innocare Pharma Tech Co Ltd | Liver Neoplasms; Solid tumours | Details |
| SC-0011 | SC-0011 | Phase 1 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours | Details |
| HS-236 | HS-236 | Phase 1 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Solid tumours | Details |
| SY-4798 | Phase 1 Clinical | Shouyao Holding (Beijing) Co Ltd | Solid tumours | Details | |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Head and Neck Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Roblitinib | FGF-401; NVP-FGF401 | Phase 2 Clinical | Novartis Pharma Ag | Solid tumours; Neoplasms; Carcinoma, Hepatocellular | Details |
| ABSK-011 | ABSK-011 | Phase 2 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours; Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| Fisogatinib | BLU-554; CS-3008 | Phase 2 Clinical | Blueprint Medicines Corp | Carcinoma, Hepatocellular | Details |
| Rogaratinib | BAY-1163877 | Phase 2 Clinical | Bayer AG | Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Non-Small-Cell Lung | Details |
| Aldafermin | M-70; NGM-282 | Phase 2 Clinical | Ngm Biopharmaceuticals Inc | Liver Cirrhosis, Biliary; Constipation; Non-alcoholic Fatty Liver Disease; Renal Insufficiency; Irritable Bowel Syndrome; Fibrosis; Cholangitis, Sclerosing; Diarrhea; Hepatic Insufficiency; Diabetes Mellitus; Malabsorption Syndromes | Details |
| H3B-6527 | H3B-6527 | Phase 1 Clinical | H3 Biomedicine Inc | Liver Neoplasms; Carcinoma, Hepatocellular | Details |
| SYHX-2005 | SYHX-2005; SYHX2005 | Phase 1 Clinical | Cspc Ouyi Pharmaceutical Co Ltd | Solid tumours; Neoplasms | Details |
This web search service is supported by Google Inc.